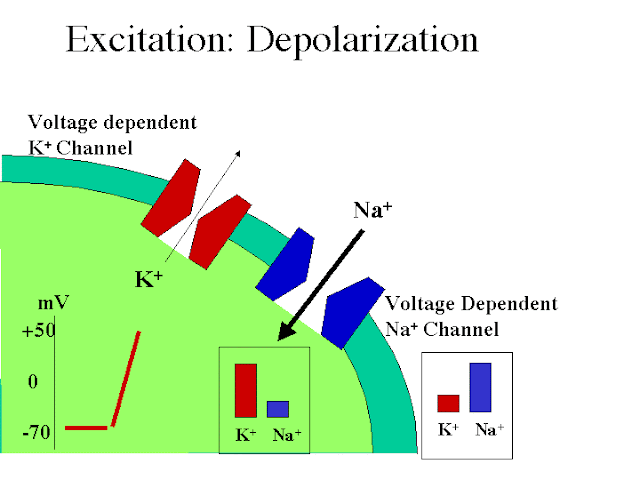
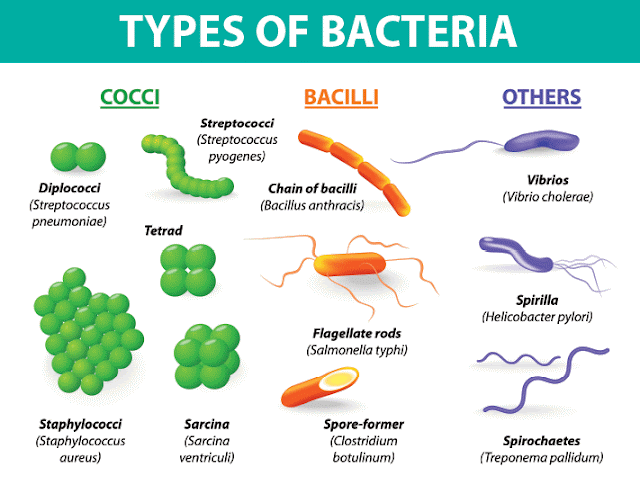
Intramolecular Catalysis:
The acceleration of a chemical transformation at one site of a MOLECULAR ENTITY through the involvement of another FUNCTIONAL (“catalytic”) GROUP in the same molecularentity, without that group appearing to have undergone change in the reaction product. The use of the term should be restricted to cases for which analogous INTERMOLECULAR CATALYSIS by CHEMICAL SPECIES bearing that catalytic group is observable. Intramolecular catalysis can be detected and expressed in quantitative form by a comparison of the reaction rate with
that of a comparable model compound in which the catalytic group is absent, or by measurement of the EFFECTIVE MOLARITY of the catalytic group.